By Dr. Evanguelos Xylinas
Based on Keynote-45 (NCT02256436), pembrolizumab was been approved for the treatment of locally advanced or metastatic UC that progresses during or after a platinum-containing regimen. The updated results after >3 y of follow-up since the last patient was randomized were presented at ESMO19.
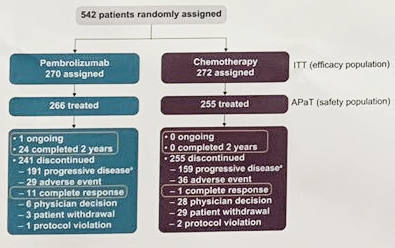
The Keynote-45 study included patients with histologically or cytologically confirmed UC, progression after platinum-containing chemo, ECOG PS 0-2, measurable disease per RECIST v1.1, or ≤ 2 lines of systemic therapy were eligible. Pts were randomly assigned 1:1 to receive pembro 200 mg Q3W or investigator’s choice of paclitaxel 175 mg/m2 Q3W, docetaxel 75 mg/m2 Q3W, or vinflunine 320 mg/m2 Q3W. Primary end points: OS and PFS (RECIST v1.1, blinded central review). ORR and duration of response (DOR) were key secondary end points.
As of Nov 30, 2018, among 542 enrolled pts (pembro, 270; chemo, 272), median (range) follow-up was 40.9 mo (36.6-48.9). Median OS was significantly longer with Pembrolizumab vs chemotherapy (10.1 vs 7.2 mo; HR, 0.72; P = 0.0003). OS rates at 24 mo were 26.9% with Pembrolizumab and 14.2% with chemotherapy. 36-mo OS rates were 20.7% and 11.0%, respectively. OS benefit with Pembrolizumab vs chemotherapy was maintained regardless of age, ECOG PS, prior therapy, liver metastases, baseline hemoglobin, time from last chemotherapy, histology, risk factors, and chemo choice. Median PFS was similar between arms (2.1 vs 3.3 mo; HR, 0.96; P = 0.32). Median DOR for responders was longer with Pembrolizumab than chemotherapy (29.7 mo [1.6+ to 42.7+] vs 4.4 mo [1.4+ to 42.8+]), and a greater proportion of responses lasted ≥24 mo (56.8% vs 28.3%, Kaplan-Meier). Median survival follow-up for responders was 39.6 mo for Pembrolizumab and 17.7 mo for chemotherapy. ORR was higher with Pembrolizumab vs chemo (21.1% vs 11.0%). Fewer patients given Pembrolizumab vs chemotherapy experienced a treatment-related adverse event of any grade (62.0% vs 90.6%) and a grade ≥3 adverse event (16.9% vs 50.2%).
Expert’s comment:
Within 3 years of follow-up, Pembrolizumab continued to exhibit an OS benefit and superior safety vs chemotherapy in pts with locally advanced or metastatic UC that progressed during or after platinum-based chemotherapy. Moreover, patients’ responders to Pembrolizumab experienced a durable response with a median time of response of more than 2 years. This longer follow-up, establishes Pembrolizumab as the standard of care 2nd line therapy in locally advanced or metastatic UC that progresses during or after a platinum-containing regimen.
Read the abstract of Keynote-045 here.