Background
D is an anti–PD-L1 antibody approved in the US for patients (pts) with locally advanced or metastatic urothelial carcinoma (UC) who progressed on or after platinum-based chemotherapy (CT). DANUBE is a phase 3 study to evaluate D, with or without T (an anti–CTLA-4 agent), as a first-line treatment for metastatic UC (NCT02516241).
Methods
Eligible pts were ³18 years of age with an ECOG PS of 0 or 1 and previously untreated, unresectable, stage IV UC. Pts were randomized 1:1:1 to D (1.5 g IV q4w), D+T (D 1.5 g IV q4w + T 75 mg IV q4w for up to 4 doses, followed by D 1.5 g IV q4w), or CT (gemcitabine + cisplatin or carboplatin) for up to 6 cycles, until disease progression or unacceptable toxicity. Randomization was stratified by cisplatin eligibility, PD-L1 status (high [≥25% of tumor and/or tumor-associated immune cells staining positive] vs low [<25% of both tumor and immune cells staining positive]), and presence/absence of visceral metastases. Dual primary endpoints compared overall survival (OS) for (1) D vs CT in pts with high PD-L1 expression and (2) D+T vs CT in the ITT population. Minimum follow-up time (from the date the last pt was randomized) was 34 months.
Results
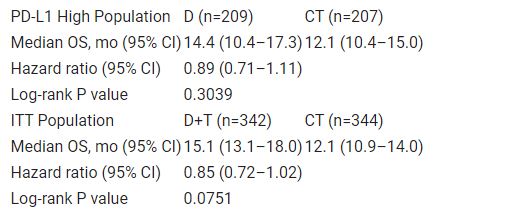
A total of 1032 pts were randomized. Median OS was not significantly different between D and CT among pts with high PD-L1 expression, nor between D+T and CT in the ITT population (Table). Treatment-related adverse events of grade 3–4 occurred in 14%, 28%, and 60% of pts in the D, D+T, and CT arms, with deaths possibly related to treatment in 0.6%, 0.6%, and 0.3% of pts, respectively.
Table: 697O
Conclusions
While a trend towards improved OS was observed with D vs CT in the PD-L1 high population and with D+T vs CT in the ITT population, statistical significance was not reached. Additional analyses are ongoing to characterize D and D+T efficacy/safety in different pt subgroups.