Background
C, a multitargeted receptor tyrosine kinase inhibitor (TKI), promotes an immune-permissive environment that may enhance response to immune checkpoint inhibitors (ICIs). COSMIC-021, a multicenter phase 1b study, is evaluating C + A (anti‒PD-L1 therapy) in various solid tumors (NCT03170960). C + A demonstrated encouraging clinical activity in cohort 2 of COSMIC-021 in patients (pts) with UC previously treated with platinum-containing chemotherapy (chemo) (Pal S et al. ASCO 2020. Abstract 5013). Outcomes of C + A from 3 other UC cohorts (C3, C4, C5) are presented.
Methods
Pts with inoperable locally advanced/metastatic UC with transitional cell histology and ECOG PS 0‒1 were eligible. Pts enrolled in C3 and C4 had no prior therapy and were cisplatin-based chemo ineligible (C3) or eligible (C4). C5 enrolled pts with one prior ICI and no prior VEGFR-TKI therapy. Pts received C 40 mg PO QD and A 1200 mg IV Q3W. CT/MRI scans were performed Q6W for first year and Q12W thereafter. The primary endpoint is objective response rate (ORR) per RECIST v1.1 by investigator. Other endpoints: safety, duration of response (DOR), PFS, and OS.
Results
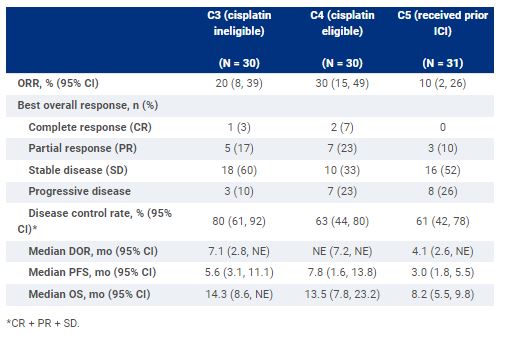
Thirty pts each were enrolled in C3 and C4, and 31 in C5. Baseline characteristics for C3, C4, and C5, respectively: median age, 74 y, 66 y, 68 y; male, 67%, 73%, 55%; ECOG PS 1, 63%, 57%, 74%; lung/liver metastasis; 33%/17%, 40%/20%, 58%/23%; ≥3 tumor sites, 30%, 43%, 45%; bladder as primary site, 67%, 70%, 71%. As of Nov 30, 2021, the median follow-up for C3, C4, and C5 was 27.9, 19.1, and 32.9 mo, respectively, with 1, 6, and 1 pts on treatment. C + A demonstrated clinical benefit across all cohorts (Table). Most common treatment-related adverse events (TRAEs) of any grade across C3, C4, and C5, respectively, were diarrhea (43%, 33%, 35%), nausea (27%, 17%, 26%), fatigue (27%, 27%, 48%), and decreased appetite (33%, 27%, 39%); grade 3/4 TRAEs occurred in 63%, 43%, and 45%, and there was no grade 5 TRAE.
Conclusions
C + A demonstrated encouraging clinical activity with manageable toxicity in inoperable locally advanced/metastatic UC as first-line systemic therapy in cisplatin-based chemo eligible/ineligible pts and as second- or later line in pts who received prior ICI. Clinical trial information: NCT03170960.