Background
First-line (1L) therapy for cisplatin (cis)-ineligible patients (pts) with mUC includes alternative chemotherapy or anti-PD-(L)1 monotherapy for PD-L1 positive tumors. The pan-FGFR inhibitor ERDA has established clinical benefit in 2L mUC for pts with targetable FGFRa. ERDA combined with the anti-PD-1 CET could expand 1L options for pts with FGFRa. The Ph 1b part of NORSE (NCT03473743) determined a tolerable dose of ERDA + CET in pts with 2L mUC. We report early results from the Ph 2 part of NORSE evaluating ERDA or ERDA + CET in cis-ineligible pts with 1L mUC and FGFRa.
Methods
NORSE Ph 2 is enrolling pts ≥ 18 y with mUC, select FGFRa (mutation/fusion), and measurable disease (no prior systemic therapy for mUC, cis ineligible). Pts are randomized 1:1 to receive once-daily ERDA 8 mg (with pharmacodynamically guided uptitration [UpT] to 9 mg) or ERDA 8 mg (no UpT) + IV CET 240 mg every 2 wks at cycles 1-4 and 480 mg every 4 wks thereafter. Primary end points are investigator-assessed overall response rate (ORR) per RECIST 1.1 and safety; secondary include disease control rate (DCR), time to response (TTR), and duration of response (DOR).
Results
As of July 19, 2021, 53 pts were randomized; 26 to ERDA and 27 to ERDA + CET. For ERDA vs ERDA + CET: median age was 75 vs 69 y; visceral metastases were present in 54% vs 52%; ECOG was 0-1 in 77% vs 63%. Efficacy data in response-evaluable pts are shown in the table. The safety set comprised 24 pts who received ERDA and 24 who received ERDA + CET. The most frequent treatment-emergent adverse events were hyperphosphatemia (ERDA vs ERDA + CET, 58% vs 58%), stomatitis (63% vs 54%), and diarrhea (50% vs 42%).
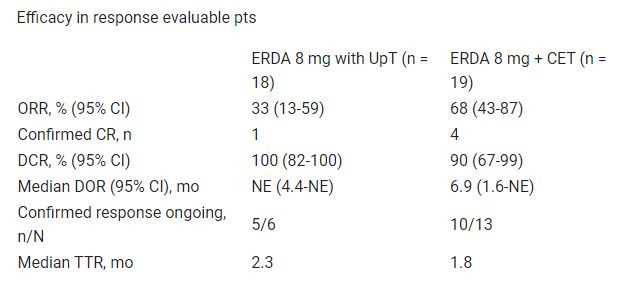
Conclusions
ERDA + CET showed clinically meaningful responses in cis-ineligible patients with 1L mUC and FGFRa. Safety was generally consistent with ERDA alone. Enrollment is ongoing.
Clinical trial identification
NCT03473743.
Editorial acknowledgement
Medical writing assistance was provided by Benjamin Ricca, PhD, Autumn Kelly, MA, and Khalida Rizi, MPharm, PhD, of Parexel, and was funded by Janssen Global Services, LLC.
Legal entity responsible for the study
Janssen Research & Development, LLC.
Funding
Janssen Research & Development, LLC.
Disclosure
T.B. Powles: Financial Interests, Personal, Research Grant: AstraZeneca; Financial Interests, Personal, Other, Honoraria/Consultant fees: Astellas; Financial Interests, Personal, Other, Honoraria/Consultant fees: AstraZeneca; Financial Interests, Personal, Other, Honoraria/Consultant fees: Bristol Myers Squibb; Financial Interests, Personal, Other, Honoraria/Consultant fees: Eisai; Financial Interests, Personal, Other, Honoraria/Consultant fees: Exelixis; Financial Interests, Personal, Other, Honoraria/Consultant fees: F. Hoffmann-La Roche; Financial Interests, Personal, Other, Honoraria/Consultant fees: Johnson & Johnson; Financial Interests, Personal, Other, Honoraria/Consultant fees: Incyte; Financial Interests, Personal, Other, Honoraria/Consultant fees: Ipsen; Financial Interests, Personal, Other, Honoraria/Consultant fees: Merck; Financial Interests, Personal, Other, Honoraria/Consultant fees: Pfizer; Financial Interests, Personal, Other, Honoraria/Consultant fees: Seattle Genomics; Financial Interests, Personal, Other, Travel reimbursement: AstraZeneca; Financial Interests, Personal, Other, Travel reimbursement: F. Hoffmann-La Roche; Financial Interests, Personal, Other, Travel reimbursement: Ipsen; Financial Interests, Personal, Other, Travel reimbursement: Pfizer; Financial Interests, Institutional, Research Grant: Astellas; Financial Interests, Institutional, Research Grant: AstraZeneca; Financial Interests, Institutional, Research Grant: Bristol Myers Squibb; Financial Interests, Institutional, Research Grant: Eisai; Financial Interests, Institutional, Research Grant: EMD Serono; Financial Interests, Institutional, Research Grant: Exelixis; Financial Interests, Institutional, Research Grant: F. Hoffmann-La Roche; Financial Interests, Institutional, Research Grant: Johnson & Johnson; Financial Interests, Institutional, Research Grant: Ipsen; Financial Interests, Institutional, Research Grant: Merck; Financial Interests, Institutional, Research Grant: Novartis; Financial Interests, Institutional, Research Grant: Pfizer. V. Moreno: Financial Interests, Personal, Advisory Role: Bayer, Bristol Myers Squibb. Y. Vano: Financial Interests, Personal, Advisory Role: BMS, MSD, Pfizer, Novartis, Ipsen, Roche, Astellas, Sanofi, Janssen. Y. Loriot: Financial Interests, Personal, Advisory Role: Janssen, Astellas Pharma, Roche, AstraZeneca, MSD Oncology, Clovis Oncology, Seattle Genetics, Bristol Myers Squibb; Financial Interests, Institutional, Advisory Role: Janssen, MSD Oncology; Financial Interests, Personal, Other, Travel, Accommodations, Expenses: Astellas, Pharma, Janssen Oncology, Roche, AstraZeneca, MSD Oncology, Clovis Oncology, Seattle Genetics, Bristol Myers Squibb; Financial Interests, Personal, Other, Honorria: Sanofi, Pfizer; Financial Interests, Institutional, Funding: Sanofi, Jnssen Oncology, MSD Oncolofy, AstraZeneca, Clovis oncology, Exelixis, Boehringer Ingelheim, Incyte, Pfizer, Oncogenex, Medivation, CureVac, Nektar. T.W. Kang: Financial Interests, Personal, Full or part-time Employment: Chonnam National University Medical School, Chonnam National University Hospital; Financial Interests, Personal, Advisory Role: Janssen Pharmaceuticals, Astellas Pharma; Financial Interests, Personal, Research Grant: Alvogen Korea. M. Tammaro: Financial Interests, Personal, Full or part-time Employment: Janssen Pharmaceuticals. A. O’Hagan: Financial Interests, Personal, Full or part-time Employment: Janssen Pharmaceuticals . M. Hosseini: Financial Interests, Personal, Full or part-time Employment: Janssen Pharmaceuticals. S. Triantos: Financial Interests, Personal, Full or part-time Employment: Janssen Pharmaceuticals. H. Chhabra: Financial Interests, Personal, Full or part-time Employment: Janssen Pharmaceuticals. A. Santiago-Walker: Financial Interests, Personal, Full or part-time Employment: Janssen Pharmaceuticals. A.O. Siefker-Radtke: Financial Interests, Personal, Advisory Role: Janssen, Merck, NCCN, Bristol Myers Squibb, AstraZeneca, Bavarian Ndic, Seattle Genetics, Nektar, Genentech, EMD Serono, Mirati Therapeutics, Basilea; Financial Interests, Personal, Other, Patents, Royalties, Other Intellectual Property: Methods of Characterizing and treatng molecular subsets of Muscle-Invsive Bladder Cancer; Financial Interests, Personal, Funding: NH, Michael and Sherry Sutton Fund for Urothelial Cancer, Janssen, Takeda, Bristol Myers Squibb, BioClin Therapeutics, Nektar, Merck Sharp & Dohme, Basilea. All other authors have declared no conflicts of interest.