Background
IMvigor130 met its co-primary endpoint of PFS (HR, 0.82 [95% CI: 0.70, 0.96]) for Arm A (atezo + PBC) vs C (PBO + PBC; Grande ESMO 2019; Galsky Lancet 2020). Atezo + PBC was well tolerated. Here we present PROs (Arm A vs C) to better evaluate the overall treatment (tx) benefit of adding atezo to PBC.
Methods
1213 eligible patients (pts) were randomized to Arm A, B (atezo monotherapy) or C. Atezo or PBO was given 1200 mg IV on day (D) 1 of each 3-wk tx cycle; PBC included gemcitabine 1000 mg/m2 IV on D1 and 8 + carboplatin AUC 4.5 or cisplatin 70 mg/m2 IV on D1. EORTC QLQ-C30 was completed q3w during tx, at tx discontinuation and ≈ q3 mo during survival follow-up. Descriptive analyses of scale scores (range, 0-100) included change from baseline (BL) summaries and time to deterioration (TTD) in all-comers in Arm A (n = 451) and C (n = 400).
Results
Completion rates were 86% at BL and > 70% until wk 66 in both arms; BL PRO scores were comparable. TTD was similar for most function/quality of life (QOL; Table) and symptom scales. HRs for symptom scales (nausea/vomiting, insomnia, appetite loss, diarrhoea, pain, constipation, fatigue, dyspnoea) ranged from 0.74 (95% CI: 0.57, 0.96; nausea/vomiting) to 1.16 (95% CI: 0.89, 1.51; dyspnoea). 12-mo deterioration-free rates favoured Arm A for nausea/vomiting, appetite loss, role function and social function. Change from BL summaries suggested similar changes (stable or marginal improvement) for both arms in most symptom, function and QOL domains through wk 51 (23% pts eligible for PRO assessments). Arm A pts sustained improved QOL above BL until wk 117 (wk 69 in Arm C).
Table: 698O
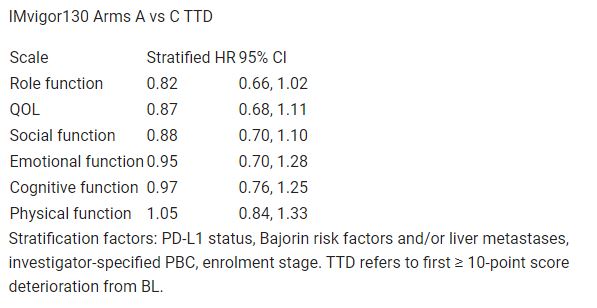
Conclusions
Atezo + PBC improved PFS vs PBO + PBC without compromising pt function or QOL. Collectively, prolonged PFS, a satisfactory safety profile and these PROs reinforce atezo + PBC as an important new 1L mUC tx option.