Research Funding
BMS, Other Foundation
Background
Transurethral resection of bladder tumor (TURBT) plus systemic therapy has been known for decades to achieve durable bladder-intact survival in a subset of patients with MIBC but efforts to advance this paradigm have been complicated by (a) lack of prospective studies exclusively testing cisplatin-based neoadjuvant chemotherapy, (b) lack of rigorous methods to define clinical complete response (cCR) and its association with long term outcomes and (c) limited understanding of the role of “salvage” cystectomy.
Methods
Eligible patients were cisplatin-eligible with cT2-T4aN0M0 urothelial bladder cancer. Patients received 4 cycles of gemcitabine, cisplatin, plus nivolumab followed by clinical restaging including urine cytology, MRI/CT of the bladder, cystoscopy and bladder/prostatic urethral biopsies. Patients achieving a cCR (normal cytology, imaging, and cT0/Ta) were eligible to proceed without cystectomy and receive nivolumab q2 weeks x 8 followed by surveillance; otherwise, patients underwent cystectomy. Coprimary endpoints included (1) cCR rate and (2) ability of cCR to predict 2-year metastasis-free survival (MFS). The key secondary endpoint was the impact of genomic alterations in baseline TURBT (TMB, ERCC2, FANCC, RB1, ATM) on performance of cCR for predicting MFS. The cCR rate coprimary endpoint, and interim analysis of 1-year outcomes, are reported.
Results
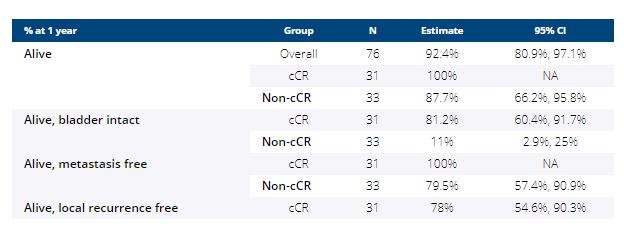
Between 8/2018-11/2020, 76 patients were enrolled at 7 sites (male 79%, median age 69; cT2 = 56%, cT3 = 32%, cT4 = 12%) and 64 (84%) have completed post-cycle 4 restaging; 31/64 achieved a cCR (48%; 95% CI 36%, 61%). The median follow-up of cCR patients is 13.7 months (range, 2.5-24 months). One cCR patient opted for immediate cystectomy (pTaN0M0). Outcomes for the entire cohort are summarized in the table below. Local recurrence has occurred in 8/31 cCR patients and 6 underwent cystectomy (pT0N0 = 1, pTaN0 = 1, pTisN0 =1, pT2N0 = 2, pT4N1 = 1). TMB ≥ 10 mut/Mb (p=0.02) or mutant ERCC2 (p=0.02) were associated with cCR or pT0.
Conclusions
TURBT + gemcitabine, cisplatin, plus nivolumab achieves stringently defined cCR in a large subset of patients with MIBC. 1-year bladder intact survival is possible though the durability of responses, and role of genomic biomarkers in management algorithms, requires longer follow-up. Clinical trial information: NCT03558087