Background
In the CheckMate 274 trial, disease-free survival (DFS) was significantly improved with nivolumab (NIVO) vs placebo (PBO) both in intent-to-treat (ITT) patients (pts) (hazard ratio [HR], 0.70; 98.22% confidence interval [CI], 0.55–0.90; P < 0.001) and in pts with tumor programmed death ligand 1 (PD-L1) expression ≥ 1% (HR, 0.55; 98.72% CI, 0.35–0.85; P < 0.001). We report results for the subgroup of pts with bladder cancer, the most predominant type of urothelial carcinoma.
Methods
CheckMate 274 is a phase 3, randomized, double-blind trial of adjuvant NIVO vs PBO in high-risk muscle-invasive urothelial carcinoma (bladder, ureter, renal pelvis) after radical resection. Pts were randomized 1:1 to NIVO 240 mg intravenously every 2 weeks or PBO for ≤ 1 year of adjuvant treatment and stratified by nodal status, prior neoadjuvant cisplatin, and tumor PD-L1 expression. Pts had radical resection ± neoadjuvant chemotherapy and were at high risk of recurrence on final pathologic staging. Primary endpoints were DFS in ITT pts and in pts with PD-L1 ≥ 1%. Non–urothelial tract recurrence-free survival (NUTRFS) was a secondary endpoint, and distant metastasis-free survival (DMFS) was an exploratory endpoint. This exploratory analysis focused on the subgroup of pts with muscle-invasive bladder cancer (MIBC) after radical resection.
Results
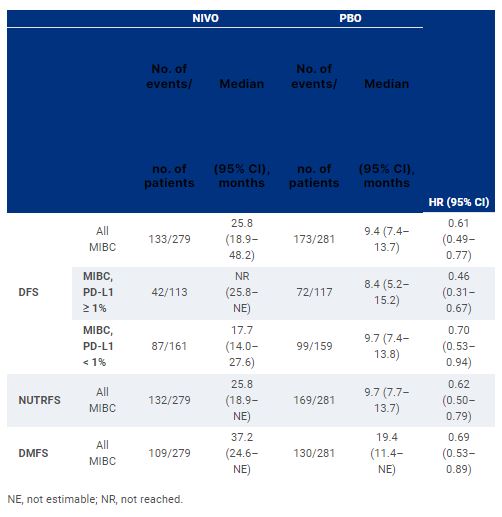
Of 709 randomized pts in the trial, 560 had MIBC (NIVO, n = 279; PBO, n = 281). With a minimum follow-up of 11.0 months, a DFS benefit was observed with NIVO vs PBO in these pts, regardless of tumor PD-L1 expression (Table). DFS probability at 12 months in all MIBC pts was 66% with NIVO and 45% with PBO. DFS was improved with NIVO vs PBO across subgroups according to age, sex, ECOG performance status, nodal status, and PD-L1 expression status. Improvement in NUTRFS and DMFS with NIVO vs PBO was also observed (Table). Grade 3–4 treatment-related adverse events occurred in 17% and 6% of pts in the NIVO and PBO arms, respectively.
Conclusions
Improvement in DFS was observed with NIVO over PBO in pts with MIBC after radical resection regardless of tumor PD-L1 expression. The DFS benefit was observed in all prespecified subgroups. These results further support adjuvant NIVO as a standard-of-care treatment for pts with high-risk MIBC after radical resection ± neoadjuvant cisplatin-based chemotherapy. Clinical trial information: NCT02632409.