Background:
Both dose-dense Methotrexate-Vinblastine-Adriamycin/doxorubicin-Cisplatin (ddMVAC) and Gemcitabine-Cisplatin (GC) are accepted neoadjuvant regimens for muscle-invasive bladder cancer (BC). We investigated COXEN, a gene expression model, as a predictive biomarker.
Methods:
Eligibility included Stage cT2-T4a N0 M0, urothelial BC (mixed histology allowed), ≥ 5 mm of viable tumor, cisplatin eligible, with plan for cystectomy. 237 patients were randomized between ddMVAC, given every 14 days for 4 cycles, and GC, given every 21 days for 4 cycles. The primary objective was to assess whether the pre-specified dichotomous treatment-specific COXEN gene expression profile is prognostic of pT0 rate or ≤ pT1 at surgery, and to assess whether COXEN score is a predictive factor between regimens and response. Logistic regression was used to model response, adjusting for stratification factors.
Results:
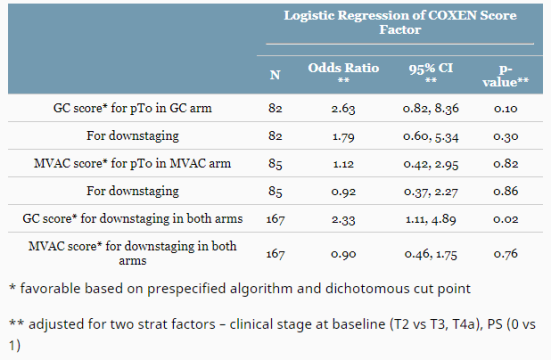
167 patients were included; the ddMVAC/GC arms had a median age of 65/64, PS = 0 in 80%/75%, Male proportion of 88%/79% and T2 stage of 87%/92%. All had at least 3 cycles of chemo and surgery/progression within 100 days of last chemo. There were favorable COXEN ddMVAC scores in 32% and GC score in 26%. The pT0 rates for ddMVAC and GC were 32% and 35%; the rates of ≤ pT1 were 55% and 49%, respectively.
Conclusion:
The COXEN scores were not significantly prognostic for response in their individual arms; The COXEN GC score was significant predictor for downstaging in pooled arms. There was no evidence of an interaction between COXEN score and regimen in predicting response. The prospective data and samples from this study will allow for further development of COXEN and other predictive biomarkers. Clinical trial information: NCT02177695.