Background
CheckMate 274 (NCT02632409), a phase 3 trial of adjuvant nivolumab (NIVO) vs placebo (PBO) in high-risk muscle-invasive urothelial carcinoma (MIUC) after radical resection, demonstrated improved disease-free survival (DFS) with adjuvant NIVO vs PBO in the intent-to-treat (ITT) and tumor PD-L1 expression ≥ 1% populations. We report exploratory analyses (minimum follow-up, 11.0 months) of associations between pre-treatment tumor and immune features and DFS to identify patients (pts) who may potentially benefit most from adjuvant NIVO.
Methods
Pts were randomized 1:1 to NIVO 240 mg IV every 2 weeks or PBO for ≤ 1 year of adjuvant treatment. Pre-treatment tumor tissue from the most recently resected site or the transurethral resection yielding MIUC diagnosis was provided for biomarker analyses. Tumor mutation burden (TMB; mutations/MB) was measured by whole exome sequencing. CD8+ immune cell infiltration was measured with digital immunohistochemistry (IHC). Gene signature scores were computed from RNA-seq data. Cox proportional hazards models were used to assess association between DFS and biomarkers (continuous scale) and estimate treatment-effect hazard ratios (HRs; NIVO vs PBO) for biomarker tertile subgroups.
Results
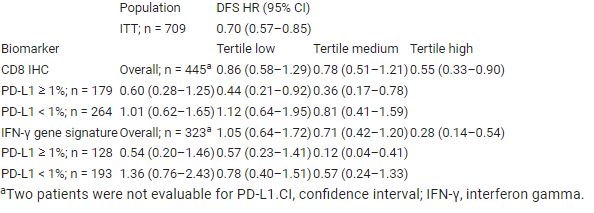
Of 709 randomized pts, 458, 445, and 323 were evaluable for TMB, CD8 IHC, and RNA-seq. TMB, and CD8 T cell infiltration and IFN-γ signature scores (Table) were positively associated with DFS in the NIVO arm. The associations appeared more pronounced in pts with PD-L1 ≥ 1%. Of these 3 biomarkers, evidence that association with DFS differed between arms was strongest for the IFN-γ signature.
Conclusions
Biomarkers associated with preexisting antitumor immunity were associated with improved DFS with NIVO vs PBO, reinforcing the mechanism for benefit with immunotherapy and extending prior findings in metastatic UC to the adjuvant setting. Multivariable modeling is ongoing. Further validation of these exploratory analyses is warranted. Table: 1737MO
Clinical trial identification
NCT02632409.