By Dr. Evanguelos Xylinas
Phase II trial of atezolizumab in BCG-unresponsive NMIBC: SWOG S1605
Non-muscle invasive bladder cancer (NMIBC) is a heterogeneous disease, with high-risk patients at an increased risk of experiencing disease recurrence and/or progression to muscle-invasive disease. To decrease this inherent risk, adjuvant instillations of BCG are currently the gold standard treatment.
Treatment options for patients with BCG unresponsive or refractory disease are under debate. The options were comprised of radical cystectomy, further intravesical therapy including intravesical chemotherapy, systemic therapy, and clinical trials. Recently, pembrolizumab was FDA-approved for this indication based on the results of the Keynote-057 trial.
Dr. Peter Black (CA) and his colleagues presented the results of the SWOG S1605 trial assessing the efficacy of another immune checkpoint inhibitor , atezolizumab, in the setting of BCG-unresponsive high-risk NMIBC.
SWOG S1605 was a phase II single-arm two cohort trial (70 patients with carcinoma in situ [CIS] and 65 with non-CIS) with histologically proven BCG-unresponsive high-risk NMIBC who were ineligible for or declined radical cystectomy. Enrolled patients received systemic atezolizumab (1200 mg IV) every 3 weeks for 1 year. The primary outcome was the pathological complete response (CR) rate at 6 months as defined by mandatory biopsy.
At ASCO 2020, the results of the cohort with CIS (with or without concomitant Ta/T1) who received at least one protocol treatment were reported. Thirty patients (41.1%, 95% confidence interval [CI] 29.7% to 53.2%) experienced complete response at 3 months and 19 patients (26.0%, 95% CI 16.5% to 37.6%) had complete response at 6 months.
AEs were identified in 61 patients (83.6%). The most frequent of these were fatigue in 36 (49.3%) patients, pruritus in 8 patients (11.0%) patients, hypothyroidism in 8 patients (11.0%), and nausea in 8 patients(11.0%). Serious AEs (Grade 3-5) occurred in 9 patients (12.3%) and there was 1 treatment-related death.
Atezolizumab showed similar efficacy with pembrolizumab in this BCG-unresponsive population, meeting the benchmark for initial CR defined by the FDA. Ongoing assessment of the duration of response will impact the implementation in daily practice.
Imvigor010: Primary Analysis from a Phase III Randomized Study of Adjuvant Atezolizumab vs Observation in High-Risk Muscle-Invasive Urothelial Carcinoma
The standard of care treatment of muscle-invasive urothelial carcinoma is the combination of neoadjuvant cisplatin-based chemotherapy and extirpative surgery (radical cystectomy). The optimal adjuvant management of the patients is not yet established (adjuvant chemotherapy or observation).
In the Imvigor010 study,Dr. Maha Hussain and colleagues evaluated the benefit of adjuvant atezolizumab by randomizing high-risk patients with muscle-invasive bladder cancer (MIBC) after surgery to either observation or q3w atezolizumab (up to 16 cycles). The primary endpoint was disease-free survival in the intention-to-treat population, and the secondary endpoint was overall survival (OS) (Figure 1).
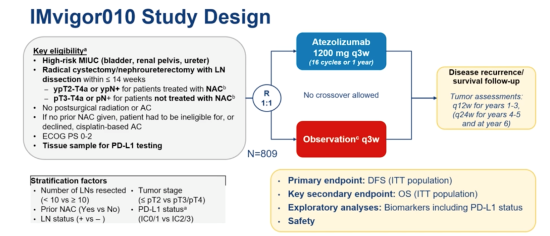
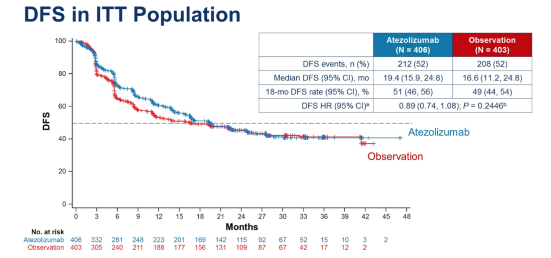
The study did not meet its primary endpoint of improved disease-free survival with adjuvant atezolizumab, regardless of PD-L1 expression within the tumour. At 21.9 months of median follow-up, the median OS in either cohort had not been reached, and there was no significant difference in survival at the data cut-off between observation and atezolizumab (Figure 2). Therefore PD-L1 therapy in the adjuvant setting after surgery is not currently recommended in unselected patients with MIBC.
Maintenance avelumab + best supportive care (BSC) versus BSC alone after platinum-based first-line chemotherapy in advanced urothelial carcinoma: JAVELIN Bladder 100
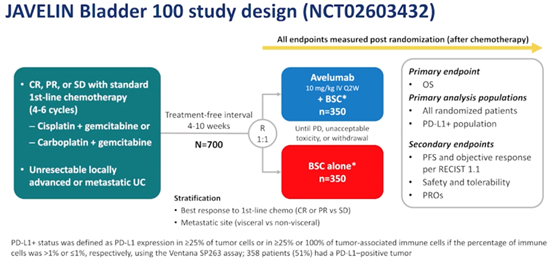
Powles et al. reported the results of the JAVELIN-100, a phase III randomized study for maintenance avelumab therapy vs best supportive care in patients who experienced disease response or stable disease from first-line platinum-based chemotherapy for metastatic urothelial cancer.
Patients had to have received at least 4 cycles of first-line chemotherapy and at minimum, have had stable disease. Within 10 weeks of completion of chemotherapy, the patients were randomized in receiving avelumab maintenance therapy or best supportive care (Figure 3). The patients were stratified by best response to first-line chemotherapy as well as metastatic site. The primary endpoint of this study was overall survival (OS) in the entire cohort and in the PD-L1 positive population.
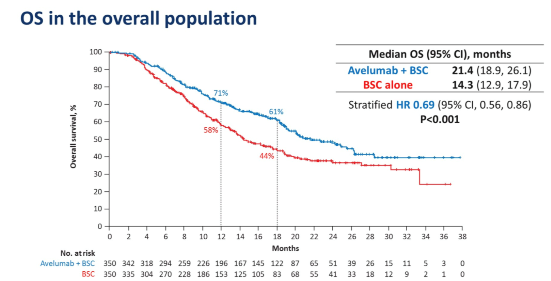
Within a median follow-up of 19.6-months, JAVELIN-100 met its primary endpoint (OS 21.4 months vs 14.3 HR 0.69 (056-0.86; p< 0.001) (Figure 4). 24% of the 350 patients in the avelumab arm were still receiving therapy vs 7% in the best supportive care arm. The median duration of treatment with avelumab was 24.9 weeks, and median duration in best supportive care arm was 13.1 weeks. Disease progression was the most likely cause for discontinuation.
JAVELIN-100 is the first switch-maintenance immunotherapy after chemotherapy trial to be positive for OS benefit in solid tumours and is likely to be an immediate practice change for urothelial cancer treatment.