By Dr. Evanguelos Xylinas
Platinum-based chemotherapy has been the standard first-line therapy for patients with metastatic urothelial cancer (mUC). Historically, response to standard of care second-line chemotherapy regimens is lower than 15%. Although immune checkpoint inhibitors (ICI) now provide another option for patients with platinum-refractory mUC, there is a clinical need for novel therapeutic agents in this disease setting. Dr. Scott Tagawa at ESMO 2019 presented the initial results from TROPHY-U-01: A Phase 2 open-label study of Sacituzumab Govitecan (SG) in patients with mUC after progression during platinum-based chemotherapy or an ICI.
SG is a Trop-2 directed antibody-drug conjugate linked to SN-38, a toxic payload that is the active metabolite of the chemotherapy drug irinotecan. Trop-2 is an epithelial cell surface antigen with high expression in mUC as well as other solid tumors. Results in mUC from a Phase I/II single-arm basket study, demonstrated an encouraging 27% overall response rate (ORR) with a PFS of 7.3 months.
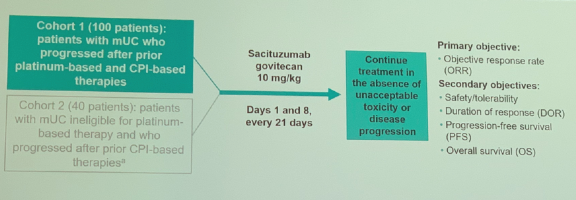
The TROPHY-U-01 study included two cohorts: cohort 1 of 100 patients with mUC who progressed after prior platinum-based chemotherapy and an ICI, and cohort 2 of 40 patients with mUC who are platinum-ineligible and progressed after an ICI. All patients were treated with SG 10 mg/kg on days 1 and 8 of a 21-day cycle until disease progression. The primary endpoint was ORR with secondary outcomes including safety/tolerability, duration of response, progression-free survival (PFS), and overall survival.
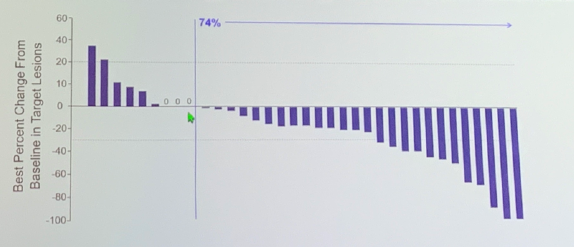
Data on Cohort 1 were presented at ESMO 2019: the median age in the cohort was 64, 80% of patients were male, the majority were white (83%) and all patients were ECOG 0-1. 63% of patients had visceral metastases with 23% of the cohort having liver metastases. The median number of prior lines of therapy was 3, ranging from 2 to 6. After a median follow-up of 4.1 months, 57% of patients continued to be on treatment. The ORR was 29% (10/35) with 6% of patients (2/35) achieving a complete response. Of the 10 patients who responded, 8 had ongoing response at the time of data collection. 74% of patients demonstrated a reduction in tumor burden by RECIST criteria.
In conclusion, TROPHY-U-01 confirmed the clinical activity of Sacituzumab Govitecan (ORR of 29%) in a heavily-pretreated population of patients with mUC post-progression on platinum-based chemotherapy and an ICI. SG was well-tolerated with a manageable safety profile. These data demonstrated that Sacituzumab Govitecan is a promising novel antibody-drug conjugate with the potential to change the treatment landscape of mUC in the third-line setting following platinum-based chemotherapy and an ICI.
Read the abstract of TROPHY-U-01 study here.