Background
The prognosis for patients (pts) with advanced UC remains poor, particularly for those unable to tolerate platinum-based chemotherapy. Defects in DNA damage repair (e.g., mutations in homologous recombination repair [HRR] genes) are common in UC and render tumor cells sensitive to poly(ADP-ribose) polymerase (PARP) inhibition. HRR gene mutations (HRRm) and PARP inhibition may enhance the antitumor response of immune checkpoint inhibitors. We conducted a randomized phase II study to evaluate D (anti–PD-L1) in combination with O (a PARP inhibitor) or placebo (P) as a first-line treatment for platinum-ineligible pts with unresectable, stage IV UC (BAYOU; NCT03459846).
Methods
Eligible pts were an age of ³18 years with an ECOG performance status (PS) of 0, 1, or 2, histologically or cytologically confirmed transitional cell carcinoma, and who had not received prior systemic therapy for unresectable, stage IV disease. Pts were randomized 1:1 to receive D (1500 mg IV q4w) plus O (orally at 300 mg BID) vs D (1500 mg IV q4w) plus O-matching placebo (P). Pts were stratified according to centrally-determined HRR status (mutant vs wild-type) and Bajorin risk index (a composite of visceral metastases and ECOG PS [0, 1 vs 2]). The primary endpoint was progression-free survival (PFS) by RECIST v1.1 (investigator assessed) in the intention-to-treat (ITT) population. Secondary endpoints included overall survival (OS) in the ITT population and PFS in the subset of pts with HRRm. The data cutoff occurred on October 15, 2020.
Results
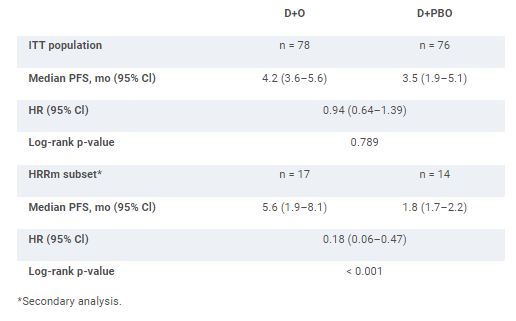
A total of 154 pts were randomized to receive D+O (n = 78) or D+P (n = 76). Among all randomized pts at baseline, 17%, 42%, and 40% had an ECOG PS of 0, 1, or 2, respectively, and 20% had an HRRm. Median PFS was not significantly different between D+O and D+P in the ITT population (Table). In the subset of pts with HRRm, median PFS was 5.6 months in the D+O group and 1.8 months in the D+P group (Table). In the ITT population, median OS (95% CI) was 10.2 months (7.0–13.9) in the D+O group and 10.7 months (7.2–17.3) in the D+P group (HR 1.07, 95% CI 0.72–1.61). Among all treated pts, grade 3 or 4 treatment-related adverse events occurred in 18% and 9% in the D+O and D+P groups, respectively, with one death due to anemia in the D+P group.
Primary and secondary PFS analyses.
Conclusions
The BAYOU study did not meet its primary endpoint. However, the results of pre-planned secondary analyses suggest a potential role for PARP inhibition in UC pts harboring HRRm. No new safety signals were observed.