Research Funding
None
Background
Bladder cancers (BC) are frequently highly mutated. Next generation sequencing (NGS) can both shed light on mutational burden and the specific alterations that provide insights into the underlying biology of individual tumors.
Methods
We retrospectively reviewed BC cases assessed with UCSF500, an institutional NGS assay that uses hybrid capture enrichment of target DNA to interrogate approximately 500 frequently mutated cancer genes. Hypermutated tumors were defined as having TMB > 10 mutations/Mb. Fisher’s exact test was used to compare patients (pts) with hypermutated (HM) and non-hypermutated (NHM) tumors.
Results
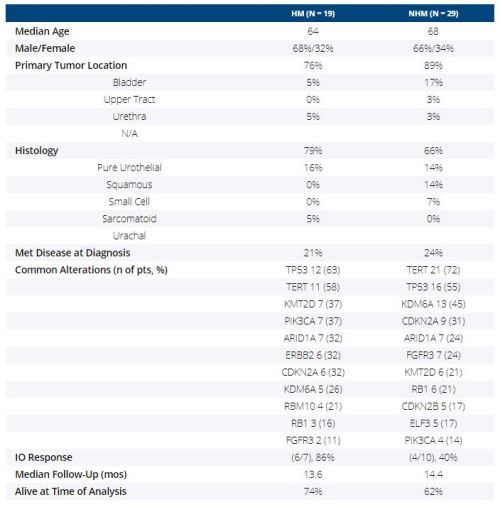
From 2015 to 2019, 74 pts with BC underwent UCSF500 testing; 48 pts were evaluable for TMB, of which 19 pts (40%) had HM tumors. 17/19 pts were evaluable for mutational signatures; all 17 had APOBEC signatures. Signatures were not assessed in NHM tumors due to low TMB. Clinicopathologic characteristics and most common alterations in the two groups are listed in the table. More HM pts had responses to immunotherapy (IO) treatment (86% vs 40%, p = 0.13).
Conclusions
In this single-institution BC cohort, HM tumors were common and APOBEC mutational signature was the common underlying biology in HM tumors. There were relevant differences in common alterations between HM and NHM tumors, including more FGFR3 mutations in NHM tumors. HM status and APOBEC signature were suggested as relevant predictive biomarkers of response to IO, which should be investigated further in larger BC cohorts.