Research Funding
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA
Background
Pembro was approvedfor cisplatin-ineligible patients with untreated advanced UC based on initial results of the phase 2 KEYNOTE-052 study (NCT02335424), which showed an ORR of 29%. Updated results after up to 5 years of follow-up are presented.
Methods
KEYNOTE-052 is a single-arm, multi-site, open-label trial. Patients had advanced or metastatic UC, were cisplatin ineligible (criteria: ECOG PS 2, CrCl ≥30 to ̃60 mL/min, grade ≥2 peripheral neuropathy/hearing loss, NYHA class III heart failure), and had not previously received chemotherapy for advanced/metastatic disease. Patients received pembro 200 mg IV Q3W until progression, unacceptable toxicity, withdrawal, or 24 mo of therapy, whichever occurred first. PD-L1 status was determined by combined positive score (CPS, number of PD-L1–staining cells [tumor cells, lymphocytes, macrophages] divided by the total number of viable tumor cells, multiplied by 100); PD-L1–positive was CPS ≥10. The primary end point was confirmed ORR (RECIST v1.1, independent central review). Key secondary end points were duration of response (DOR), OS, and safety.
Results
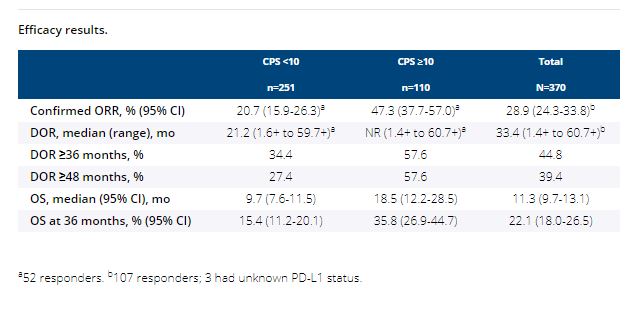
Among 370 enrolled patients, median age was 74 y, 315 (85.1%) had visceral disease, and 43 (11.6%) completed 24 mo of therapy. Median time from enrollment to data cutoff (Sep 26, 2020) was 56.3 mo (range, 51.2-65.3) for all patients and 56.0 mo (range, 51.4-65.2) for the 110 patients (29.7%) with CPS ≥10. Confirmed ORR for all patients was 28.9% (95% CI, 24.3-33.8); complete response, 9.5% (n=35); partial response, 19.5% (n=72). Median DOR was 33.4 mo (range, 1.4+ to 60.7+); 44.8% and 39.4% of patients had DOR ≥36 and ≥48 mo, (Kaplan-Meier estimates). Median OS was 11.3 mo (95% CI, 9.7-13.1); 24- and 36-mo OS rates were 31.5% and 22.1%. Patients with CPS ≥10 had better outcomes than patients with CPS <10 (Table). Treatment-related adverse events (AEs) occurred in 67.3% of patients; 21.1% of treatment-related AEs were grade ≥3, including 1 death (myositis).
Conclusions
After up to 5 y of follow-up, pembro continued to elicit clinically meaningful, durable antitumor activity in cisplatin-ineligible patients with advanced UC. These effects were more pronounced in patients with CPS ≥10. Clinical trial information: NCT02335424