Background
PD-L1 assays use various methods (e.g., scoring tumour and/or immune cells) to predict PD-L1/PD-1 blockade outcomes. Data suggest that the clinically relevant PD-L1–expressing immune cells (IC) are dendritic cells (DCs) (Oh 2020). Here, we probed the clinical and immunologic basis underlying the trend toward favourable OS with atezolizumab alone (atezo; anti–PD-L1; Arm B) vs placebo + platinum/gemcitabine chemotherapy (chemo; Arm C) in patients (pts) with high PD-L1 by SP142 in the Ph 3 IMvigor130 trial (ITT: HR, 0.68, 95% CI: 0.43, 1.08; cisplatin ineligible: HR, 0.53, 95% CI: 0.30, 0.94) (Galsky ASCO-GU 2021; 31 May 2019 cutoff).
Methods
This post hoc analysis studied associations between 2 PD-L1 immunohistochemistry tests (VENTANA SP142 and Dako 22C3) and OS in pts with archival tumours (biomarker-evaluable [BioEval] pts) in IMvigor130 Arms B and C (14 Jun 2020 cutoff). Assay cutoffs were SP142 IC ≥5% (IC2/3)/<5% (IC0/1) and 22C3 combined positive score (CPS) ≥10/<10. Samples were also stained for IC subtypes.
Results
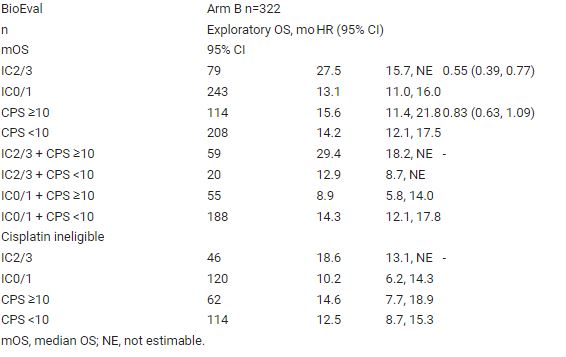
Demographics and OS of BioEval (n=627) and ITT (n=719) pts were similar. In deconvoluting the cellular components associated with PD-L1 staining, we found that SP142 preferentially co-localised with DCs (DC-LAMP, a DC-specific marker) as opposed to other myeloid subsets. Arm B OS by SP142 and/or 22C3 is shown in the table. Longer OS was associated with SP142 IC2/3 + 22C3 CPS ≥10 tumour status. Median OS was shortest in pts with tumours staining for 22C3 but not SP142 (SP142 IC0/1 + 22C3 CPS ≥10; Table).
Conclusions
This IMvigor130 exploratory analysis showed different OS trends with different PD-L1 assays. PD-L1–expressing DCs may underlie the longer OS with atezo in SP142 IC2/3 vs IC0/1 tumours in pts with mUC. These findings reinforce model system data demonstrating the particular importance of PD-L1–expressing DCs. Table: 1735O
Clinical trial identification
NCT02807636.