By Dr. Xylinas
The recently-concluded ESMO Virtual Congress 2020 delivered the latest and most relevant updates in bladder cancer, notwithstanding the online format.
Several randomised trials in the first-line metastatic setting explored immunotherapy alone; in combination with chemotherapy; or as switch maintenance after chemotherapy response has been designed.
The IMvigor130 trial, reported at ESMO 2019, randomised platinum-eligible patients with advanced or metastatic urothelial carcinoma (UC) to anti-PD-L1 monotherapy with atezolizumab, atezolizumab + platinum/gemcitabine chemotherapy, or placebo + platinum/gemcitabine therapy.
This trial showed a progression-free survival (PFS) benefit for the addition of atezolizumab to platinum/gemcitabine in the intention to treat arm, but overall survival data from this comparison are not mature.
Ajjai Alva (Ann Arbor, MI, United States of America)
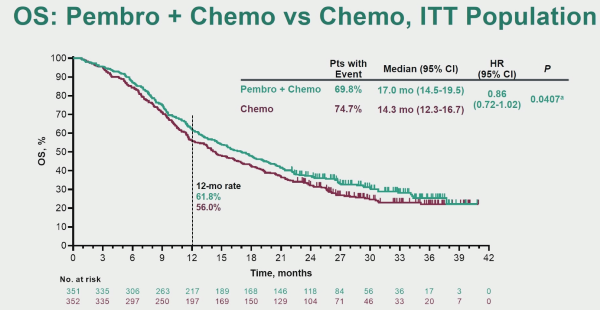
KEYNOTE-361 is a randomised, open-label, phase-3 trial of pembrolizumab alone or combined with platinum-based chemotherapy vs chemotherapy as the first-line treatment for patients with advanced or metastatic UC.
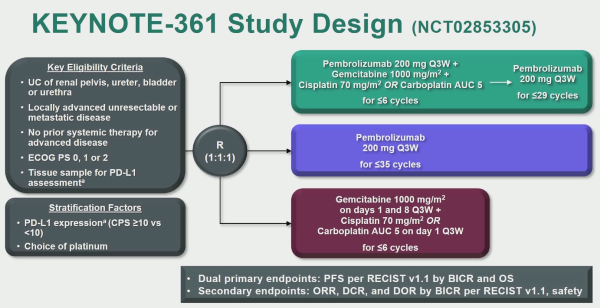
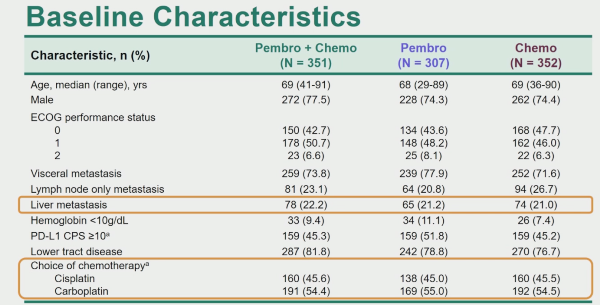
The first analysis comparing PFS in patients treated with pembrolizumab + chemotherapy versus chemotherapy alone with the intention to treat (ITT) population demonstrated a slight improvement in favour of pembrolizumab + chemotherapy, but this did not reach the pre-specified statistical significance.
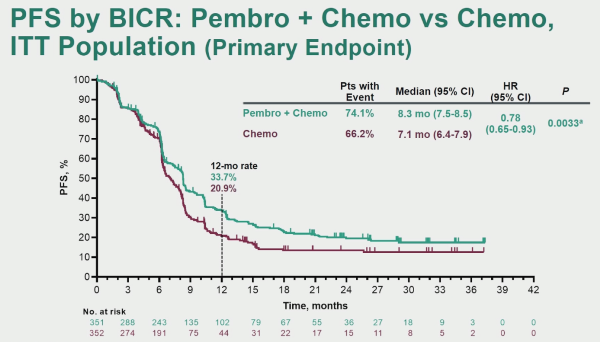
When assessing the overall survival (OS), there was an advantage to pembrolizumab + chemotherapy, but this did not reach the required statistical significance level.
Thomas B. Powles (London, United Kingdom)
Powles et al. reported the first analysis of the DANUBE study, a randomised phase-3 trial that investigated durvalumab monotherapy versus durvalumab + tremelimumab versus platinum-based chemotherapy in the first-line metastatic setting.
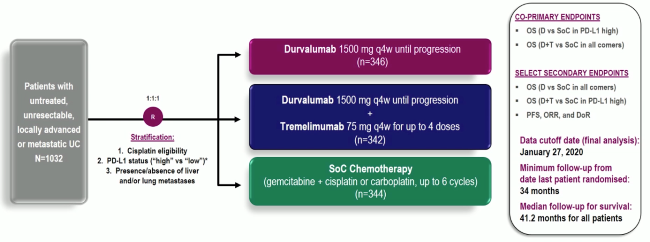
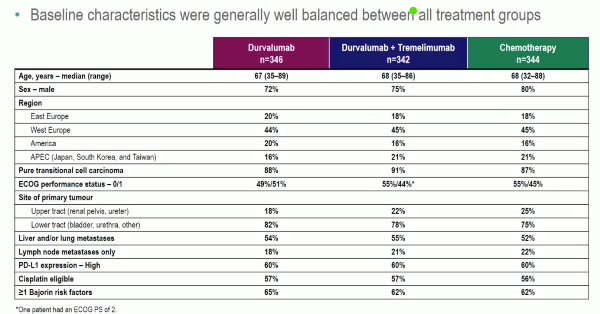
Unfortunately, the trial did not show an OS benefit survival benefit in the intention to treat the population for combination durvalumab and tremelimumab therapy compared to chemotherapy (HR: 0.85, 0.72-1.02, p=0.075), nor for durvalumab monotherapy in the PD-L1+ population compared to chemotherapy (HR: 0.89, 0.71-1.11, p=0.30).
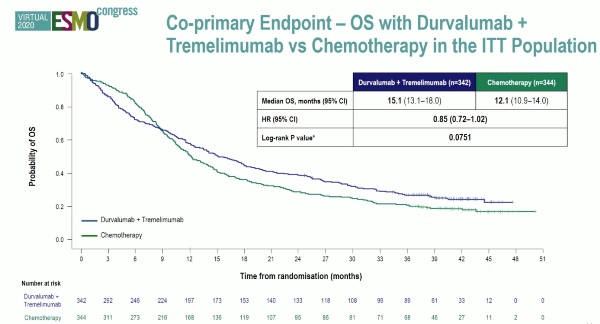
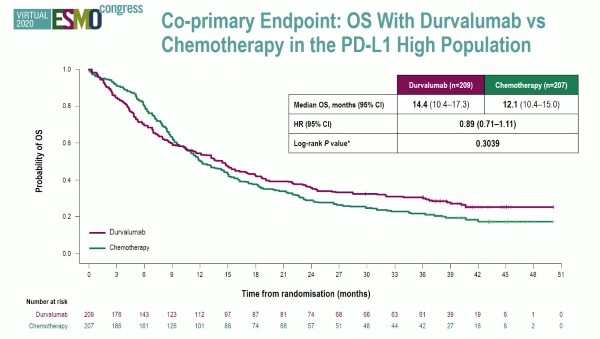
Both the KEYNOTE-361 and DANUBE studies were negative for their primary endpoints which highlighted the challenges of trial design and therapy optimization in the first-line setting for advanced UC in platinum-eligible patients.
Aristotelis Bamias (Athens, Attiki, Greece)
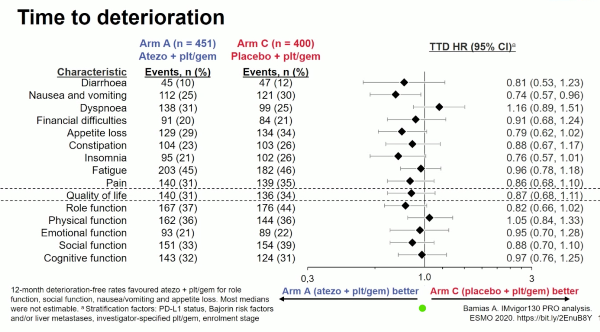
Bamias et al. reported patient-reported outcomes analyses from Arm A (combination immunotherapy and platinum chemotherapy) versus Arm C (placebo + platinum chemotherapy) of the IMvigor130 study.
The IMvigor130 study is a phase-3, randomised placebo-controlled trial investigating 3 arms with atezolizumab (anti-PD-L1 antibody) versus combination atezolizumab and platinum chemotherapy versus chemotherapy alone, as first-line treatment for advanced UC, that reported PFS benefit without increasing the toxicity.
To assess patient-reported outcomes (PROs), the investigators utilised the EORTC QLQ-C30 scales of global health, function, and cancer-related symptoms together as described below.
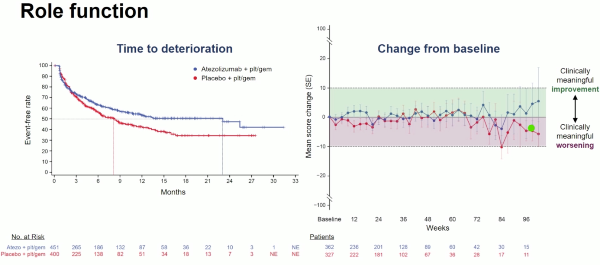
Questionnaires were administered on day 1 of each treatment cycle, within 30 days of treatment discontinuation, and every 3 months after treatment discontinuation. The addition of atezolizumab to chemotherapy did not compromise the PROs. There was a trend to an increase of the time to deterioration of quality of life and several functions including emotional, social and cognitive in favour of the atezolizumab combination arm.