By Dr. Benjamin Pradere (FR) and Dr. Evanguelos Xylinas (FR)
It was again a fascinating congress and a pleasure to cover the new edition of the ESMO Congress 2021 for you. Although the congress was virtual, we were able to feel the excitement of all these new studies and data in the field of onco-urology.
Regarding urothelial carcinoma, some really interesting studies were highly expected and did not disappoint such as VESPER and NORSE.
Although little was really game-changing in the field of non-muscle-invasive bladder cancer (NMIBC), we will report some of the most interesting results at the different stages of the natural history of bladder cancer.
Neoadjuvant setting AURA & VESPER: practice-changing?
Cisplatin-based neoadjuvant chemotherapy followed by radical cystectomy is the standard of care for non-metastatic MIBC patients based on a modest survival benefit correlated with pathological complete response.
Dr. Maria Nieves Martinez Chanza (BE) presented the first results of the AURA trial, which evaluated avelumab as a neoadjuvant therapy in platinum-eligible and ineligible patients with MIBC. The neoadjuvant studies demonstrated a promising antitumor activity of single-agent immune checkpoint inhibitors, with <ypT2N0 rates of 39-56% and, in combination with chemotherapy, <ypT2N0 rates of 56-69%. The preliminary data from the AURA trial assessed preoperative avelumab with two cisplatin-based regimens in the cisplatin-eligible cohort.
The AURA trial is a prospective, multicentre, randomised, phase-II trial involving patients with cT2-4aN0-2M0 bladder carcinoma. Cisplatin-eligible patients received cisplatin-gemcitabine plus avelumab or dose-dense MVAC plus avelumab (1:1). The study schema for AURA was as follows:
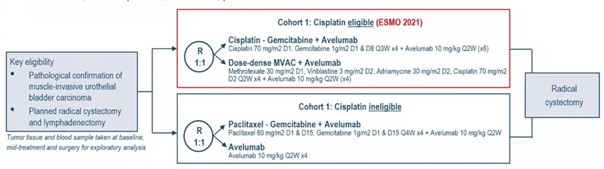
The primary endpoint was a pathological complete response (ypT0/isN0) with the objective of showing a pathological complete response rate of > 25% (90% power reached in case of a pathological complete response rate of > 45%) in each arm. An interim analysis with a two-step design was conducted after 28 patients were evaluable per arm. Secondary endpoints were a pathologic downstaging rate (<ypT2N0) and related to safety. At the interim-analysis-data cut-off, 56 cisplatin-eligible patients were evaluable. As for the cisplatin-gemcitabine + avelumab arm (n=28), the median age was 69 years (range: 41- 81), 64% were male, 64% were cT2N0, and 7% were cT4N0. As for the dose-dense MVAC + avelumab arm (n=28), the median age was 62 years (range: 51-77), 79% was male, 61% was cT2N0, and 7% was cT4N0. Six patients did not undergo surgery but were included in the intention-to-treat analysis. In the cisplatin-gemcitabine + avelumab arm, 54% of the patients met the primary endpoint of a pathologic complete response, compared to 61% in the dose-dense MVAC + avelumab arm.
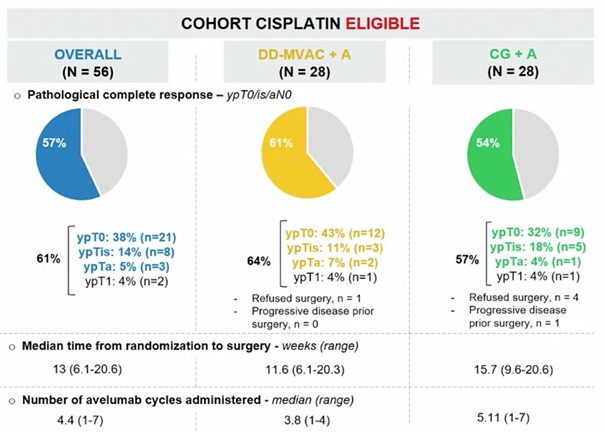
The most common grade 3/4 adverse events were thrombocytopenia (29%), acute kidney injury (18%), neutropenia (14%), and anaemia (13%).
Prof. Christian Pfister (FR) presented the results of the GETUG/AFU VESPER V05 phase-III trial, which assessed dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin (dd-MVAC) compared to gemcitabine and cisplatin (GC) as perioperative chemotherapy for patients with MIBC undergoing radical cystectomy (NCT 01812369). A total of 500 patients were randomised across 28 centres in France. Patients received either four cycles of GC every three weeks or six cycles of dd-MVAC every two weeks before surgery (the neoadjuvant group) or after surgery (the adjuvant group). The trial primarily assessed progression-free survival (PFS) at three years.
Among the 500 patients included, 437 patients (88%) received neoadjuvant chemotherapy. Compared to their planned treatment courses, 60% of the patients received the planned six cycles in the dd-MVAC arm and 84% received four cycles in the GC arm. Following chemotherapy, 91% and 90% of the patients underwent surgery in the dd-MVAC and GC arms, respectively. In terms of pathologic response, evidence of organ-confined disease following neoadjuvant chemotherapy (< ypT3N0) was observed more frequently in patients who had received neoadjuvant dd-MVAC compared to those who had received GS (77% vs 63%, p=0.001).
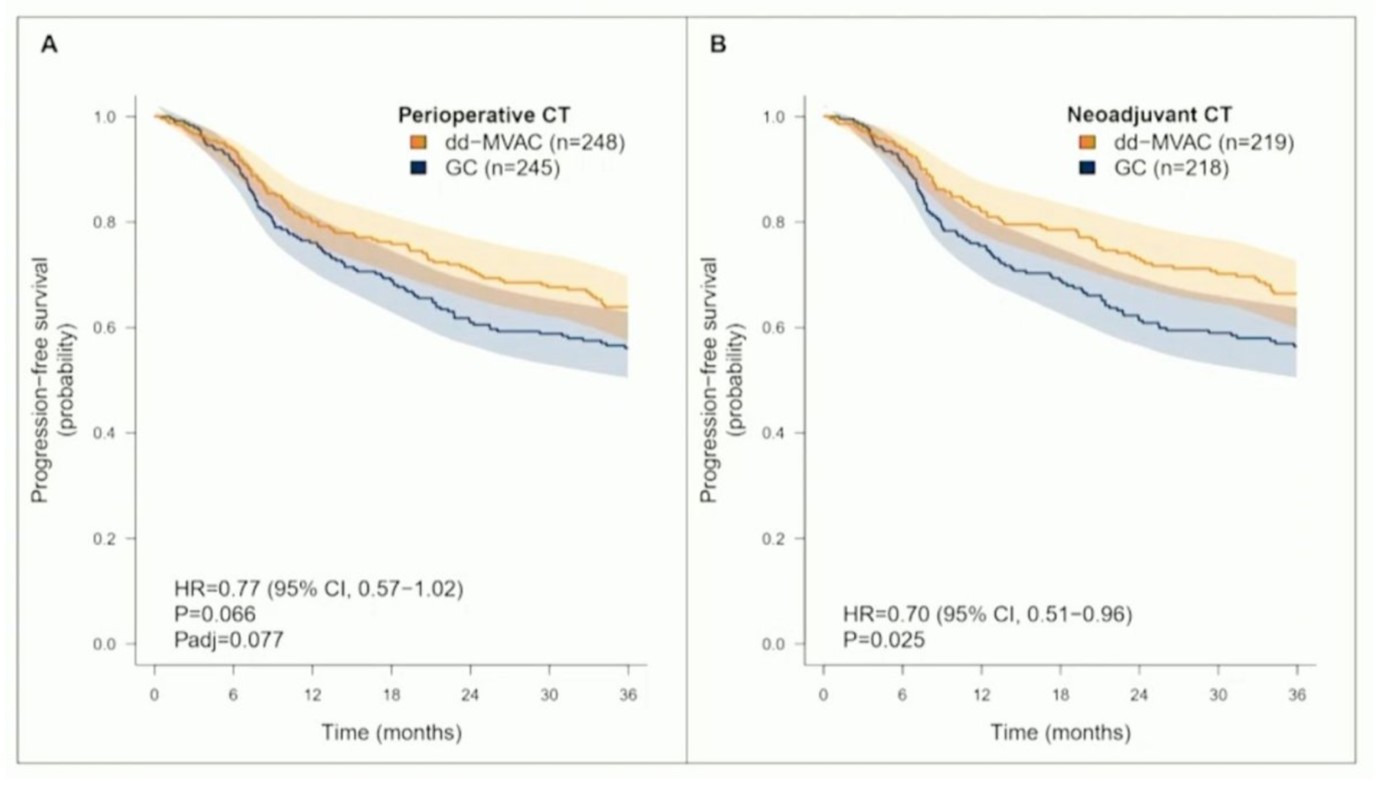
Considering the perioperative setting of the VESPER trial, the three-year progression-free survival of patients in the dd-MVAC arm had improved compared to those receiving GC (64% vs 56%, HR=0.77, p=0.066). Considering the two treatment approaches separately, in the neoadjuvant group three-year progression-free survival was significantly higher in the dd-MVAC arm compared to the GC arm (66% vs 56%, HR=0.70, p=0.025). However, in the adjuvant group the results were inconclusive due to the limited number of patients included (n=56). Additionally, assessing the overall survival in the overall study cohort led to the conclusion that the results had failed to meet a statistical significance (HR 0.74, 95% CI 0.55-1.00) while among those receiving neoadjuvant chemotherapy, dd-MVAC had significantly improved the overall survival (HR 0.66, 95% CI 0.47-0.92).
First-line treatment for mUC, the NORSE study: Are FGFR inhibitors ready for prime time?
The results of several first-line, cisplatin-ineligible trials involving FGFR inhibitors and immunotherapy were eagerly awaited: NORSE (FGFR3 mutation/fusions, erdafitinib + cetrelimab versus erdafitinib), FORT-2 (FGFR1/3 mRNA overexpression, rogaratinib + atezolizumab versus atezolizumab + placebo), and FIGHT-205 (FGFR mutation/fusions, pemigatinib + pembrolizumab versus pemigatinib versus standard of care).
At the ESMO Congress, Dr. Srikala Sridhar (CA) presented the phase-II NORSE study that compared erdafitinib and erdafitinib plus cetrelimab as a first-line therapy in patients with cisplatin-ineligible metastatic urothelial carcinoma (mUC) and FGFR alterations.
It has been shown that FGFR alterations are present in up to 20% of the patients with mUC. So far, erdafitinib is the only FGF inhibitor that is FDA-approved for mUC (for patients progressing after platinum chemotherapy). Some data have shown that FGFR and PD-(L)1 inhibitors may be effective when they are associated.
One of the co-primary endpoints was the safety. Both arms had the same safety profile, with the most common AE being Hyperphosphatemia, which might be a biomarker of treatment response. The other primary endpoint was the objective response rate (ORR). The combination therapy showed a higher ORR of 68% compared to erdafitinib alone (33%) as well as a higher complete response rate (CRR) (21% vs. 6%). The results are promising as the ORR is among the highest in this setting as well as the CRR is the highest ever reached.
Nevertheless, this was a small cohort, and a longer follow-up is necessary before giving any strong conclusions. Having said that, these results will continue to empower the benefit of combination therapy.
This study, among many others, underlined the difficulties of conducting a biomarker-guided trial. There is still a need to improve screening (many patients are needed to be screened), and the insufficient availability of tissue of sufficient quantity and quality is also an important issue. All in all, biomarker-guided studies are more than promising but still need to be refined to improve the enrolment without delaying treatments.
Second-line treatment for mUC
Dr. Sarmad Sadeghi (US) presented a phase-II trial of the combination of pembrolizumab (P) and sEphB4-HSA (B4) in patients previously treated for mUC. EphrinB2 is a transmembrane protein expressed in capillary endothelial during development (during the embryonic period primarily) which ceases to be expressed in adults. EphB4 is a related high-affinity cognate receptor with similar longitudinal expression patterns. These two molecules are expressed in UC and are associated with a negative prognosis. A soluble receptor (sEphB4-HSA) may block the interaction of these two molecules by occupying EphrinB2. This agent would be synergistic with anti-PD-1/PD-L1 antibodies to improve anti-tumour activity.
In this phase-II trial, the researchers included patients with platinum-refractory mUC who had not received prior anti-PD-1/PD-L1 therapy. Patients received pembrolizumab 200mg IV q3 weeks + B4 10mg/kg IV weekly in a 21-day cycle until progression (PD) or unacceptable toxicities.
The co-primary endpoint was tolerability and overall survival (OS) while secondary endpoints included PFS, duration of response (DOR), and ORR. 70 patients were enrolled with a median age of 67. The majority had BC (80%) and visceral metastases (89%) and had received prior cisplatin-based chemotherapy (74%). In 39% of the patients, variant histology was detected. The EphrinB2-positive subgroup (46/70; 66% of the overall cohort) demonstrated similar characteristics.
The combination with Pembrolizumab has shown promising results. The ORR was 37% in the overall cohort with a CRR of 16%. In the EprhinB2-positive cohort, the ORR was 52% with a CRR of 24%. Of note, with a median follow-up of 23 months the median DOR was not reached in the overall cohort nor in the EphrinB2-positive cohort.
The authors have shown that responses occurred early in the treatment (mostly in the first two-four months) and that 70% of the responders maintained said responses beyond 24 months.
Regarding survival, there was a greater activity in the EphrinB2-positive cohort. The median PFS was 4.1 months in the overall cohort compared to 5.7 in EphrinB2-positive patients. Similarly with OS, which was 14.6 months in all patients compared to 21.5 months in EphrinB2-positive patients. Regarding safety, the combination therapy has an acceptable toxicity profile. The most common adverse event was hypertension, which occurred in 74% of the patients and was Grade 3 in 45% of patients.
In conclusion, combination of sEphB4-HSA and pembrolizumab demonstrated synergistic activity and was well tolerated. The pre-specified subgroup analysis of EphrinB2-positive patients demonstrated greater activity in this biomarker-positive population. EphrinB2 might be a biomarker for best responders to sEphB4-HSA-based therapy.